Representing quantum chemical systems
In this section, we introduce the MolecularOrbitals object, which is what represents a quantum chemical system such as a molecule in QURI Parts.
The MolecularOrbitals is a protocol class that is defined to hold:
- number of electrons in the system.
- number of spatial orbitals in the system.
- of the system.
- molecular orbital coefficient (mo coefficient).
In this tutorial, we specifically focus on PySCFMolecularOrbitals, which is what represents a molecule in QURI Parts with PySCF input. We will also introduce the ActiveSpaceMolecularOrbitals object, which holds the active space information of the molecule.
Defining the Molecules and Molecular Orbitals
Now, let's first build a water molecule with PySCF and create a PySCFMolecularOrbitals.
from pyscf import gto, scf
mole = gto.M(atom="O 0 0 0; H 0.2774 0.8929 0.2544; H 0.6068, -0.2383, -0.7169")
mf = scf.RHF(mole).run(verbose=0)
The PySCFMolecularOrbitals is created with a pyscf.gto.Mole object and an array that represents the mo coefficient.
from quri_parts.pyscf.mol import PySCFMolecularOrbitals
h2o_mo = PySCFMolecularOrbitals(mole, mf.mo_coeff)
print(f'Number of electrons: {h2o_mo.n_electron}')
print(f'Number of spatial orbitals: {h2o_mo.n_spatial_orb}')
print(f'Spin of the molecule: {h2o_mo.spin}')
print(f'MO coefficients:\n {h2o_mo.mo_coeff.round(3)}')
#output
Number of electrons: 10
Number of spatial orbitals: 7
Spin of the molecule: 0
MO coefficients:
[[ 0.994 -0.233 -0. 0.103 0. -0.13 0. ]
[ 0.026 0.838 0. -0.535 -0. 0.863 -0. ]
[ 0.003 0.093 -0.131 0.572 0.636 0.552 -0.212]
[ 0.002 0.069 0.45 0.424 -0.388 0.408 0.728]
[-0.002 -0.049 0.386 -0.299 0.667 -0.289 0.625]
[-0.006 0.158 0.446 0.283 0. -0.788 -0.828]
[-0.006 0.158 -0.446 0.283 0. -0.788 0.828]]
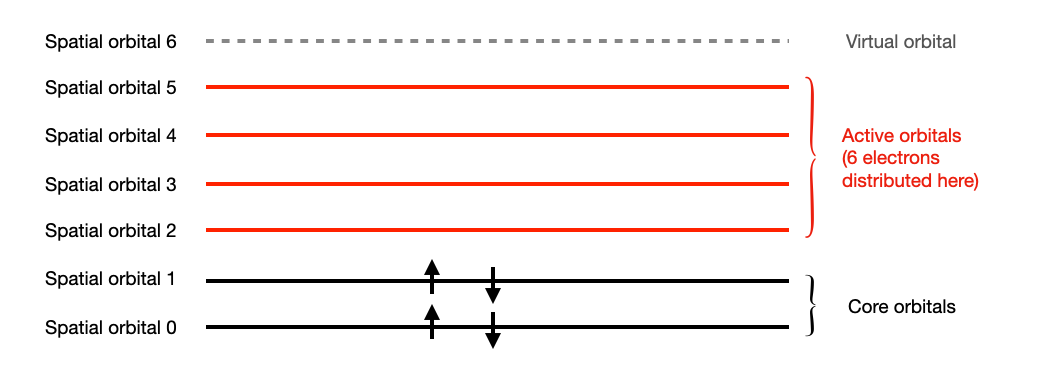
Specifying active space configuration
Sometimes, it is enough to consider the transition between a subset of the spatial orbitals. This is equivalent to assigning an active space configuration to the system. In this case, the spatial orbitals are partitioned into 3 classes.
- core orbitals: Spatial orbitals with 2 electrons.
- active orbitals: Orbitals where the electrons are allowed to freely transit from and to.
- virtual orbitals: Spatial orbitals with no electrons.
The electrons are also partitioned into 2 sets:
- core electrons: electrons that live in the core orbitals.
- active electrons: electrons that live in the active orbitals.
In QURI Parts, an active space configuration is specified by the ActiveSpace object.
from quri_parts.chem.mol import ActiveSpace, cas
active_space = ActiveSpace(n_active_ele = 6, n_active_orb = 4)
# or equivalently:
active_space = cas(n_active_ele = 6, n_active_orb = 4)
print(active_space)
#output
ActiveSpace(n_active_ele=6, n_active_orb=4, active_orbs_indices=None)
We can then assign the active space to a molecule by creating an ActiveSpaceMolecularOrbitals. It will provide detailed information about the molecule after the active space is assigned to it.
from quri_parts.chem.mol import ActiveSpaceMolecularOrbitals
asmo = ActiveSpaceMolecularOrbitals(h2o_mo, active_space)
print("The molecule configuration with active space assigned to it:\n")
print(asmo)
#output
The molecule configuration with active space assigned to it:
n_electron: 10
n_active_ele: 6
n_core_ele: 4
n_ele_alpha: 3
n_ele_beta: 3
n_spatial_orb: 7
n_active_orb: 4
n_core_orb: 2
n_vir_orb: 1
To obtain the list of core and active orbitals, we can use the get_core_and_active_orb method.
core_orbital_list, active_orbital_list = asmo.get_core_and_active_orb()
print("core spatial orbitals:", core_orbital_list)
print("active spatial orbitals:", active_orbital_list)
#output
core spatial orbitals: [0, 1]
active spatial orbitals: [2, 3, 4, 5]
We can also obtain the spatial orbital's type with the orb_type method.
print("Type of sptial orbital 0:", asmo.orb_type(0))
print("Type of sptial orbital 2:", asmo.orb_type(2))
print("Type of sptial orbital 8:", asmo.orb_type(8))
#output
Type of sptial orbital 0: OrbitalType.CORE
Type of sptial orbital 2: OrbitalType.ACTIVE
Type of sptial orbital 8: OrbitalType.VIRTUAL